Kuldeep Raizada, Ph D BCO, BADO, FAAO
Kuldeep Raizada is a Licensed Ocularist in Hyderabad, a Board Certified Ocularist (BCO) from National examination Board of ocularist’s, USA and a Board Approved Diplomate Ocularist (BADO) from American Society of ocularist’s, specializing in ocular prosthetics since 2001, Kuldeep places his emphasis on the satisfaction and well-being of every patient. His clinical skill and expertise are equally matched by his personalized care for patients and attention to detail.
Kuldeep Raizada completed his basic optometry education at Gandhi Eye Hospital, Aligarh, and has his training at L V Prasad Eye Institute, Hyderabad. where he was also Founder and Head of the Department of Ocular Prosthesis services till 2009. He completed a second fellowship, in Anaplastolgy, at MD Anderson Cancer Centre, Houston. He has also been trained by the top most ocularist and anaplastologist in United States of America.
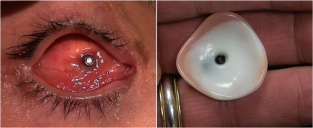
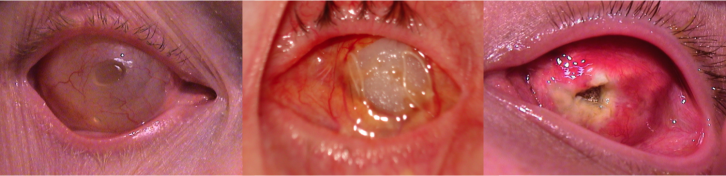
His clinical interests include ocular and facial prosthesis, particularly in pediatric patients. His research interests lie in newer advancement in development of new types of prosthesis, newer solution for ptosis corrective glasses.
Kuldeep Raizada, is Founder & Director of the International Prosthetic Eye Center since 2010, where he is practicing since 2010.
Kuldeep Raizada has been recognized by the American Society of Ocularist, USA and American Anaplastology Association,USA and by several other professional organizations, for his excellence in research and clinical practice.
Kuldeep Raizada, have completed all requirements by American Society of Ocularist, which is hard work of 14000 working hours as well extensive study for prosthetics, Hence awarded the Diplomate Ocularist from American Society of Ocularist, USA, 2012, Chicago, USA, which is the First ever received all over Asia Pacific & throughout Middle East so ever.
At present he is reviewer of several journals like Contact Lens & Anterior Eye, International Journal of Anaplastology, Oculoplasty & Reconstructive Surgery (OPRS) and Many others. He has published and presented world widely.
Fact About Kuldeep
CEO & Chairman of Akriti
Founder & Director International Prosthetic Eye Center, India
Founder of Healthcare India Magazine
Receipt of Abdul Kalam Award.
First ever Indian to received Board Certifications, from National Board of examination of ocularist, USA, He was the first One all over Asia Pacific & throughout Middle East.
Developed First Digital Dynamic Facial Eye Prosthesis
Developed physiological Near Stereo test
Developed physiological Distance Stereo test
Developed ETDRS Log Mar Vision Charts in Hindi for 4 Meters
Developed ETDRS Log Mar Vision Charts in Telegu for 4 Meters
Developed ETDRS Log Mar Vision Charts in Assamese for 4 Meters
Developed ETDRS Log Mar Vision Charts in Arabic for 4 Meters
Developed ETDRS Log Mar Vision Charts in Bangla for 4 Meters
Developed ETDRS Log Mar Vision Charts in Tamil for 4 Meters
Developed ETDRS Log Mar Vision Charts in Oriya for 4 Meters
Developed ETDRS Log Mar Vision Charts in Kannada for 4 Meters
Developed ETDRS Log Mar Vision Charts in Malayalam for 4 Meters