know more about
Dermis Fat Graft
A know how
The dermis fat graft (DFG) for an anopthalmic socket reconstruction was first described by Smith and Petrelli in 1978. It has become widely employed as a technique by which to augment orbital volume in enucleated patients.[1] The technique is used both as a primary procedure following enucleation, and as a secondary procedure for extrusion or migration of an orbital implant.[1]Inadequate replacement of a volume deficit by an orbital implant and ocular prosthesis can lead to enophthalmos and depression of the upper eyelid.[2] In addition, changes in orbital vascularity and architecture have been cited as the basis for socket contracture, though the pathophysiology continues to be a topic of debate.[3]
Among conventional autografts, DFGs are preferred for their ability to augment both volume and surface area.[3] DFGs may be harvested from the abdominal, gluteal, hip, groin, or thigh regions.[2][4] Not only do the fat and dermis provide a soft tissue and surface lining with vascular support respectively, but the graft can also act as a temporary biologic dressing.[2][4] DFGs are convenient and inexpensive, and do not cause local reactions or disease transmission.[1][4] Furthermore, chance of migration or extrusion is lower with the use of autologous tissue than with the use of artificial implants.[2]
The most commonly cited drawback of the procedure is an unpredictable rate of subsequent fat atrophy, more commonly in adults.[5] A prospective study by Bhattacharjee et al demonstrated recurrence of socket contracture with prosthesis extrusion in 4 out of 12 cases undergoing DFG, of which 11 of 12 patients were adults.[3] In comparison, Essuman et al reported a graft failure rate of only 7% in a study of 15 children.[6]
Patient Selection
Preoperative Evaluation
Surgical management is guided by patient age and history, orbital implant type, degree and duration of implant exposure, and defect size.[1][2] Larger defects are typically repaired with DFGs, but the procedure may not be appropriate for severely contracted sockets.[1][7] If available, axial length measurement of the healthy eye prior to enucleation or evisceration is obtained.[2] Preoperative inspection of the socket, superior sulcus, palpebral aperture, and lid is performed, and the level of socket contracture is graded.[3] Scar tissue in the conjunctiva, lid, and socket should be noted, and the graft site is examined for damage and poor vascular support, which can exacerbate fat atrophy.[2][3] Both recipient and donor sites should be free of infection prior to surgery.[2][3] Donor tissue must contain sufficient underlying fat to cover the area of the defect, and is ideally harvested from a region that is hairless, not subject to pressure, and easily hidden under swimwear.[2]
Vascular Support
Though DFGs are traditionally not recommended for atrophy and fibrosis secondary to ocular radiotherapy due to concerns of poor anastomosis between irradiated tissues and the graft, good results may still be achieved with hypercorrection of the donor tissue, and good intraoperative hemostasis.[5] Similarly, orbits that have suffered significant trauma, especially from chemical burns, and patients with systemic vascular disease, should receive careful consideration due to increased risk of graft failure secondary to a nonviable recipient bed.[7]
Children
DFGs may be particularly suitable for children since the graft grows with the surrounding orbital tissue. Herer and Katowitz described in 1998 that young children under age 4 were noted to have hypertrophy of the DFG that required debulking in some patients. .[5] In a prospective case series analyzing Ghanaian children between the ages of 9 months and 10 years with follow-up ranging from 3-54 months, volume augmentation of the graft and good facial symmetry were achieved in 14 out of 15 children.[6]
Indications
One of the main indication for a Dermis fat grafts is congenital anophthalmia.[1][2][3] The procedure is also indicated for complications of secondary anophthalmia following occurrence of trauma, corneal ulcer, malignancy or intraocular tumor with or without radiotherapy, glaucoma, endopthalmitis, painful blind eye, and others.[1] In a retrospective review of 41 patients by Aryasit and Preechawai, the 3 major indications for secondary DFG included:
Exposure of orbital implant (32%)
Extrusion of orbital implant (27%)
Volume insufficiency with shallow fornix (24%).[1]
Others have also indicated good success using DFG for:
Relative Contraindications
DFG may be used in these circumstances but may have limited success:
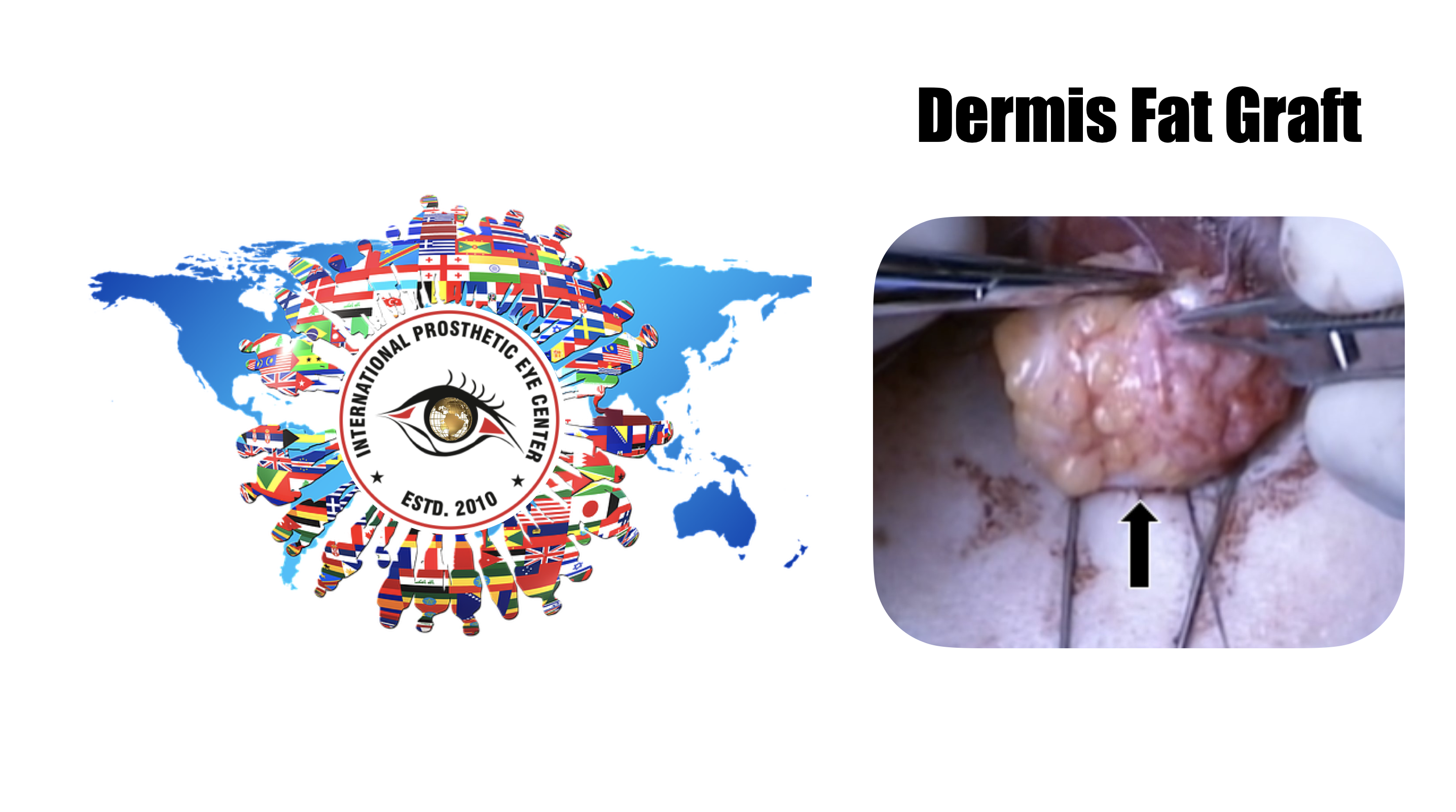
Surgical technique
An ellipsoid approximately 20 mm in diameter is placed in the lower abdominal quadrant or flank, This areas are less prone to be tender, and are non-weight bearing. Some surgeons prefer harvesting the DFG from the buttocks or lateral portion of the thigh. The ultimate diameter of the harvested graft most not exceed 25mm.
A #15 blade is then used to make an incision along the marking through the epidermis.
The #15 blade is then used to excise the epidermis from the underlying dermis. The epidermis is best removed with the dermal tissue in situ. The epidermis is excised so that keratin is not produced in the socket.
Holding the #15 blade perpendicular to the dermal face, an incision is made along the dermis to the underlying fat following the ellipse. Holding the blade perpendicularly is important in harvesting an adequate size composite-graft that is slightly wider at the base away from the dermal side. Beveling the blade will yield a conical-shaped graft with inadequate fatty tissue.
Steven scissors or Metzembaum scissors are then used to bevel out to excise as much fat as possible.
It is important to harvest more fat that what you think you will need since you will lose approximately 50% of your fat volume as things heal.
The graft can then be placed in moist saline gauze or in physiologic saline solution until needed.
The incision is then closed with deep interrupted 4-0 monocryl sutures. Vicryl sutures could also be used if preferred.
The skin can then be closed with a 5-0 or 4-0 prolene suture.
The edges of the skin should be everted. In this case a running horizontal mattress suture is placed.
Antibiotic ointment is then be placed over the incision, a Telfa dressing and a pressure bandage are applied for a week.
The graft is then transferred to the anophthalmic socket, and each of the muscles that have been tagged with the double armed 5-0 vicryl sutures are sutured to the edge of the dermis. It should be very difficult to get all of the fat into the socket.
The conjunctival edges are then sutured to the edge of the dermis with 6-0 vicryl suture, in a running or interrupted fashion. It is important to make sure that the edges of the conjunctiva are not buried or an inclusion cyst may later develop. In the following 4 weeks approximately, the dermis should become epithelialized by migration of cells across the graft from the conjunctival edges.
At the conclusion of the case a large conformer is placed. A temporary suture tarsorrhaphy can often be placed. The eye is then patched for a week. . [8] [9]
Postoperative care
Regarding the post-operative care for the surgical site from where the graft was obtained, tends to heal in the same fashion as other wounds approximated by primary intention. Some surgeons place patient who undergo autogenous tissue grafts in oral cephalosporins for 5-7 days. Graft site hematomas have been reported, and should be dealt in a conservative manner. If the surgical site is under tension, it would be advisable to release the sutures, and apply a compression bandage to the site. Closure can be reattempted after the swelling has diminished. [9]
After appropriate layered closure has been achieved at the socket, after implantation of the DFG, a conformer should to placed, as well as antibiotic ointment. The conformer will prevent extrusion of the graft , as well as proper maintenance of the fornices for a future ocular prosthesis to be fitted, usually by 6 weeks.
Complications
At the site from which the graft if harvested:
In the socket where the DFG is implanted:
Shore et al. discuss their experience following sixty consecutive cases of dermis fat graft in an enophthalmic socket. Shore et al. report conjunctival ulceration in seven patients. Ten cases resulted in enophthalmos, given the reabsorption and atrophy y of the fat graft. Two patients developed keratinized sockets with chronic discharge and desquamation. Three patients required excision of conjunctival granulomas. One patient developed a primary graft infection. A donor site hematoma occurred in one patient. Secondary surgical intervention was required in ten patients. [10]
Nine complications in eight patients were managed in the office; five complications in four patients were observed and subsequently resolved without surgical intervention. Most complications occurred in patients with severely traumatized sockets who had undergone extensive earlier ocular surgery, or who had a systemic disease contributing to defective wound healing. [11]
In another report by Quaranta-Leoni et al. describing their experience with DFG in 22 patients, they describe only one complication in a child with exuberant fat growth of the implant. The reported differences in complication rates between Shore et al. and Quaranta-Leoni et. al might be due to differences in technique. [12